- Products
- New Products
- Featured Products
- Color and Print Themes
- Blood Pressure Measurement
- NIBP / Vital Sign Monitors
- Digital Aneroid Sphygmomanometers
- Palm Aneroid Sphygmomanometers
- Pocket Aneroid Sphygmomanometers
- Pro's Combo Sphygmomanometers
- Multicuff Sphygmomanometers
- Clock Aneroid Sphygmomanometers
- Mercury Sphygmomanometers
- Home Blood Pressure Measurement
- Adcuff +
- Gauges
- Bulb & Valves
- Disposable Cuffs
- Reusable Cuffs & Bladders
- Sphygmomanometer Accessories
- Sphygmomanometer Parts
- Caseware
- CPR / Airway
- EENT
- Instruments & Accessories
- Laryngoscopes
- Penlights
- Pulse Oximeters
- Stethoscopes
- Thermometry
- Vital Signs Monitors
- Solutions
- About ADC
- Learning Center
- Support
- Blog
- Contact
Connector Update

Affecting: Diagnostix Brand Clock Aneroids and Adcuff Brand Inflation Systems
Affecting: Diagnostix Brand Clock Aneroids and Adcuff Brand Inflation Systems
Due to new regulatory guidance* from the FDA, we are changing to bayonet connectors on all Diagnostix brand clock aneroids and Adcuff brand inflation systems. The new regulations are designed to reduce the risk of misconnections between medical devices or accessories.
Beginning September 1, 2022:
- ADC brand clock aneroids (models 750W, 750D, and 752M) will be fitted with a female bayonet connector, item #8974F, on the coiled tubing.
- Adcuff brand inflation systems (models that start with 865) will be fitted with a male bayonet connector, item #8974M.
Are the New Connectors Compatible?
If you've ordered a new clock aneroid and new inflation systems, both will arrive with the updated connector style and can be used together immediately. Nothing else is required.
.jpg)
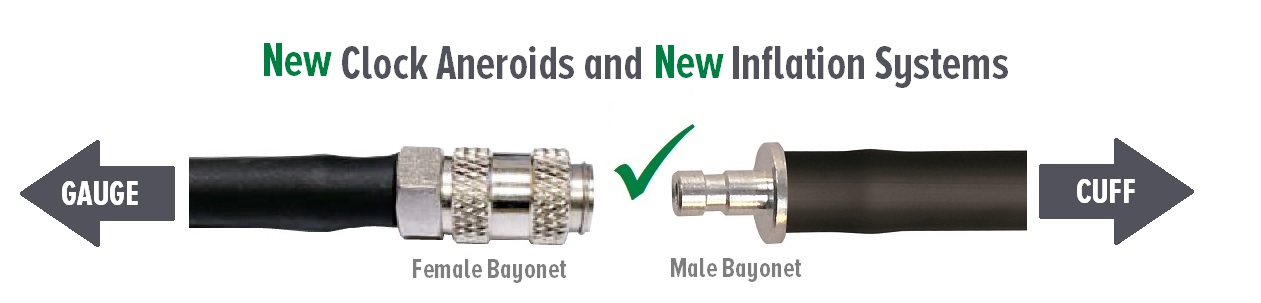
If you've ordered new inflation systems to use with a legacy clock aneroid instrument, you'll need to convert one of the connectors.
.jpg)
How Do I Convert a Connector?
It's easy, and you have two options that will allow you to use a new inflation system with a legacy clock aneroid instrument:
OPTION 1: Replace the connector on the new inflation system
During the transition period, you can replace the connector on your new inflation system with one that's compatible with a legacy gauge. For several months, ADC will be including a replacement legacy connector and instructions (#891FKIT) so you can quickly retrofit your cuff in the field. Check your inflation system packaging for this part.
OPTION 2: Add an adapter to your legacy clock aneroid
If you prefer to use the new inflation systems as is, we offer a simple adapter that you can connect to the coiled tubing on the clock aneroid. Contact your ADC sales rep, dealer, or our Customer Service team and ask for part #8974FKIT.
*For a technical explanation of the guidelines informing this change, you can read the Consensus Standard and the Informational Overview on the FDA's web site.

.jpg)
.jpg)
.jpg)